A problem that can’t be sugar coated
Background
With the burgeoning prevalence of diabetes mellitus in Australia and many other countries, there has been an accompanying increase in blood tests for its diagnosis and treatment. One of the commonest tests used is for determining the average amount of sugar in a person’s blood over the past several months, by measuring the ratio of glycated haemoglobin A1c (HbA1c) to the total haemoglobin level. Laboratories in Australia report HbA1c results according to the USA’s National Glycohemoglobin Standardization Program (NGSP), established in 1996, following the Diabetes Control and Complications Trial (DCCT) in Type 1 diabetes of 1983-1993. This standardisation protocol yields results being expressed as a mass fraction percentage, typically of the order of 4% to 9%. Australia’s national diabetes guidelines recommend HbA1c levels be kept below 7% for good control in patients with diabetes mellitus.
In recent years there has been an international trend to move to a new standardisation protocol developed by the International Federation of Clinical Chemists (IFCC). This protocol yields results, which if expressed as a percentage, would be some 15-30% less than the corresponding NGSP protocol. Thus, an NGSP reading of 6.5% would be reported as 4.8% using the IFCC protocol. To avoid confusion, most countries adopting the protocol will report HbA1c results as substance (molar) fractions in mmol/mol instead of the NGSP’s mass fraction percentages. Thus, an NGSP result of 6.5% would be reported under the IFCC protocol as 48mmol/mol.
An international consensus statement(1), formulated in 2010, recommends that:
1. HbA1c test results should be standardised worldwide, including the reference system and results reporting.
2. The IFCC reference system for HbA1c represents the only valid anchor to implement standardisation of the measurement.
3. HbA1c results are to be reported by clinical laboratories worldwide in SI (Système International) units (mmol/mol – no decimals) and derived NGSP units (% – one decimal), using the IFCC-NGSP master equation (DCCT units).
4. HbA1c conversion tables including both SI (IFCC) and NGSP units should be easily accessible to the diabetes community.
5. Editors of journals and other printed material are strongly recommended to require that submitted manuscripts report HbA1c in both SI (IFCC) and NGSP/DCCT units.
6. The reportable term for glycated haemoglobin is HbA1c, although other abbreviations may be used in guidelines and educational material (A1C).
7. The above consensus recommendations apply through 2011, when they will be discussed again at the next consensus meeting at the IDF meeting in Dubai December 2011.
The degree to which these recommendations have been adopted varies from country to country. New Zealand and the UK both moved quickly to adopt the IFCC protocol. Australia is now following suit, although the likelihood of 3. being followed is questionable.
Electronic reporting
What then does this mean for electronic reporting, and for aggregation of results across time and space? The majority of atomic structured reporting by laboratories in Australia is via HL7 version 2, using LOINC codes to identify the tests. Standards Australia have an Australian Standard AS4700.2:2007 based on HL7 v2.4 that deals with many issues of representing diagnostic results in HL7. It is supplemented by the Handbook HB262:2008 (currently being revised), which gives further guidance for implementers. Laboratories vary in their compliance to the standard. But the issues arising out of the changes to HbA1c reporting are too detailed for these standards and guides to adequately address. There is also a current push by the Australian Government to introduce a Personally Controlled Electronic Health Record (PCEHR) system which may receive pathology results in GP summaries or hospital Discharge Summaries coded by SNOMED CT terms, in HL7 CDA documents, as recommended by NEHTA. The use of either or both of these terminologies in either or both of these exchange format standards (HL7 v2, HL7 CDA) will face problems trying to embrace the change to IFCC-based reporting of HbA1c.
Terminology standards
Both LOINC and SNOMED CT are complex terminologies that aren’t comprehensively installed, let alone supported across the Australian health system. LOINC has some 55,000 codes, most of which are used for naming, and describing laboratory tests and their properties. Each code or test can have up to 55 fields of information, and the whole terminology is made available in the form of a table ( ~55,000 rows by 55 columns ). Some fields can, in turn, have multiple components and sub-components, resulting in a powerful, but complex way to describe laboratory tests. The most frequently used column of LOINC is the code column. The following 6 columns together uniquely describe each test, as illustrated below.
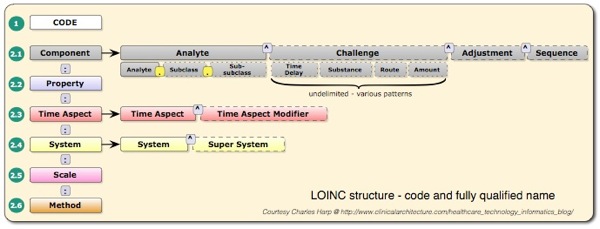
For HbA1c, the hithertofor frequently used code is 4548-4. It’s corresponding fully specified name is:
Hemoglobin A1c/Hemoglobin.total:SFr:Pt:Bld:Qn:.
Thus for this test, the analyte is Hemoglobin A1c/Hemoglobin.total, the property is Substance Fraction, the time aspect is Point in Time, the System under testing is Blood, the scale is numeric Quantity and the method is not supplied. What is actually sent in any given HL7 v2 message is anyone’s guess!! In Australia, the AS4700.2 standard recommends that Austpath codes be used. Now Austpath codes were developed on a shoestring many years ago, but through lack of governance and funding have never been developed, quality assured and improved. Unfortunately, the set as currently available, has a number of problems. It does not include values for Method. It introduces readable, Australianised “preferred names” for the tests which don’t match any names in the distributed LOINC database, nor the names used in the Royal College of Pathologists of Australasia (RCPA) Manual. For example, the LOINC long name for code 4548-4 is “Hemoglobin A1c/Hemoglobin.total in Blood“, the corresponding Austpath name is Glycosylated haemoglobin, with an analyte name of HAAEMOGLOBIN A1C (sic). So Austpath has Australianised the name, but introduced a spelling error in transcribing the analyte name from the LOINC database. The RCPA name for the corresponding test is Glycated haemoglobin – red cell.
Now the LOINC database itself is not without a number of quality and engineering issues. Firstly, the fully specified name convention promoted by the Regenstrief Institute uses a “:” character to concatentate its 6 parts. Unfortunately no mention is made in the LOINC documentation of the fact that 233 of the ~55,000 entries ( in the current 2010 database) have a “:” character in the analyte component, thus rendering the fully specified naming convention unreliable. Secondly, in the case of code 4548-4, for example, the property has been mislabelled as SFr (Substance Fraction), when in fact should be a MFr (mass fraction), albeit represented as a percentage. Recent LOINC committee discussions have suggested addressing this issue. LOINC introduced a new code for the HbA1c test standardised to the IFCC protocol. This code 59261-8, has the following fully specified name:
Hemoglobin A1c/Hemoglobin.total:SFr:Pt:Bld:Qn:IFCC method.
Note that the only difference between this and the NGSP standardised test, designated by code 4548-4 is the addition of a specific method, where none existed for 4548-4.
I won’t even bother to describe how the above issues might be addressed in SNOMED CT. At the current time, SNOMED does not have anywhere near enough information to adequately provide the level of detail that LOINC does for describing pathology tests. Furthermore, SNOMED’s custodians, the International Terminology Standards Development Organisation have neither the processes, nor the resources to currently address these issues, and I would estimate it would be many years before detailed models and synonyms for pathology tests are rationalised in SNOMED, if at all. This is despite a 2009 (commencing April 1) 4-way Memorandum Of Understanding involving the IHTSDO, the IFCC, the International Union of Pure and Applied Chemistry (IUPAC), and the The Regenstrief Institute, Inc. being drafted in 2009 for “AN OPERATIONAL TRIAL OF A DIVISION OF LABOR IN LABORATORY TEST TERMINOLOGY DEVELOPMENT INVOLVING LOINC, NPU & SNOMED CT”.
However, NEHTA went through an exercise of producing a “Reference set” for pathology results reporting which included a collection of SNOMED IDs representing test names. These names cannot readily be discerned from the published reference sets, without a SNOMED licence. I have extracted the preferred terms for the 1529 concepts, and display them here. Interested readers will note the lack of consistency in spelling across variants of haemoglobin and hemoglobin, and the lack of HbA1c as a preferred term ( also a problem with LOINC). [The spelling issue may well be resolved in current distributions]. This is not to say that SNOMED doesn’t have synonyms that include HbA1c, it is just that there is no consistent way these are described for which guidance can be given for producing useful reference sets. In fact, SNOMED has a term HbA1c measurement (DCCT aligned), a child of the concept Hemoglobin A1c measurement (procedure), but this is not included in NEHTA’s reference set. Presumably, a new concept to support the IFFC protocol will eventually be added at this level. Thus the reference sets currently published by NEHTA are inadequate for the role they are expected to play.
So we have long had a situation of confusion, just simply with the case of reporting HbA1c levels in Australia, using atomic level (computer processable) data in HL7 messages. This situation is shortly to be significantly exacerbated by the change to a new test standardisation protocol. There appears to be no organisation owning this problem. NEHTA started to own some of the problem, but by moving towards SNOMED as an overarching strategy without adequately addressing the detail of pathology test identification, it has introduced further confusion.
If we cannot reliably exchange computable information about one of the most fundamental physiological parameters regularly measured in Australia, then how will we ever deal with the overall exchange of clinical information in the broad?
How will this be solved pragmatically? Well, in the short term, I would imagine that the laboratories will continue their current practice of formulating quality reports in human readable form. They can report the new test values and reference ranges, together with the old values if need be. They can easily ( without recourse to coding schemes) transmit PIT or RTF or PDF formatted reports that humans can read, and pay lip service to the detailed atomic data needed for decision support, but which so few receiving systems can process. They can patch up any particular issue with a particular LOINC code on an ad hoc, as needs basis, if requested.
And in the longer term? Well that depends on how many years or decades one considers as longer. But it certainly won’t be adequately addressed by the PCEHR go live date of July 1, 2012.
Conclusion
Changes in the protocols for reporting, that involve changes to terminology coding systems can wreak havoc when large numbers of installed systems ( perhaps 50,000 Australia wide could be affected by these HbA1c changes ) and installed system products ( perhaps 500 separate system products/versions Australia wide ) are involved. It is not just the laboratory reporting systems that need to change – it can be every system that might need to process the data at a later date, including all clinical systems in GP clinics, hospitals, specialist research systems in hospitals and research institutions, etc.
This is not an easy problem to solve. It cannot be solved by bureaucrats simply making decrees such as “We will use SNOMED for pathology tests”, or “We will use LOINC for pathology tests”. The devil, unfortunately is in the detail.
What we need is a dedicated Clinical Data Standards organisation that will take the detail seriously. An organisation that is sufficiently funded, sufficiently resourced, sufficiently dedicated, sufficiently knowledgable and skilled, and sufficiently empowered to solve these problems. Such an organisation will have to work collaboratively with a number of stakeholders, both in Australia and overseas. It will need to commit to distributing all the artefacts required to enable interchange of clinical information for the full gamut of computer processing – including clinical decision support, data aggregation and research, electronic health record support, statistical reporting, and linking to medical knowledgebases.
With such an organisation, Australia could be a leader in improving clinical information exchange, rather than a follower of countries like New Zealand, the UK, Sweden, Denmark and Portugal.
With such an organisation, perhaps one day, we may even be able to reliably exchange computable test results for HbA1c !
References:
1. 2010 Consensus Statement on the Worldwide Standardisation of the Haemoglobin A1c Measurement
2. National Evidence Based Guidelines for Blood Glucose Control in Type 2 Diabetes
3. Clinical Architecture blog
4. LOINC Manual